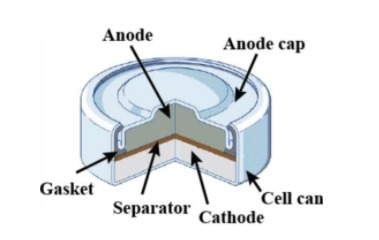
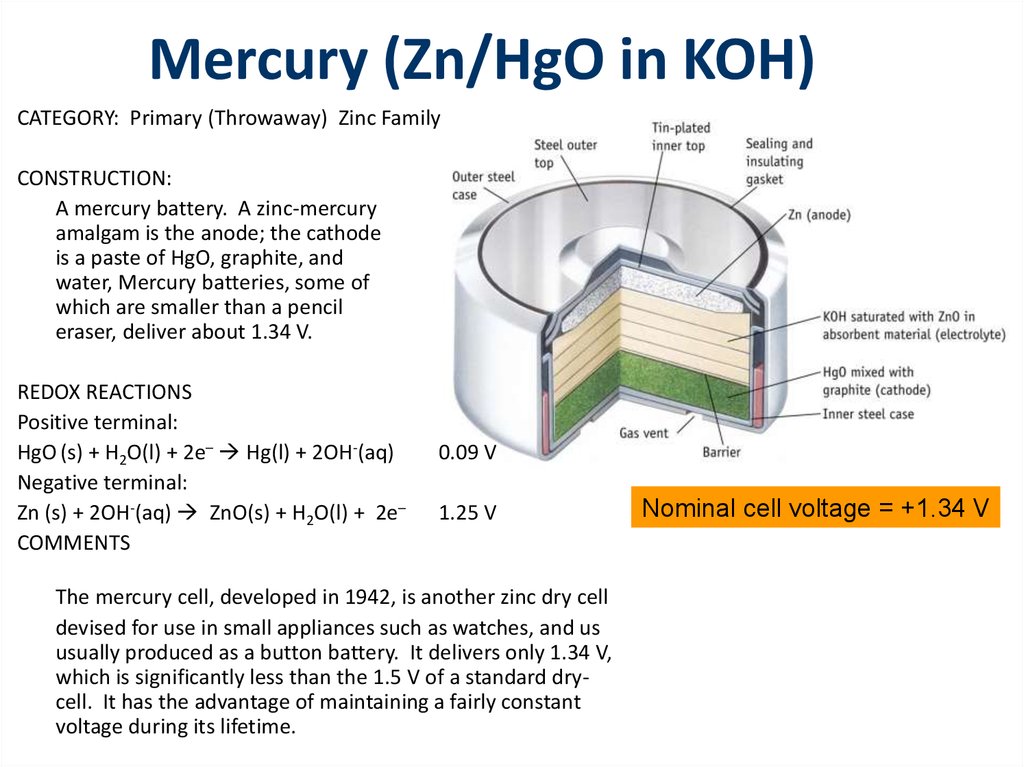
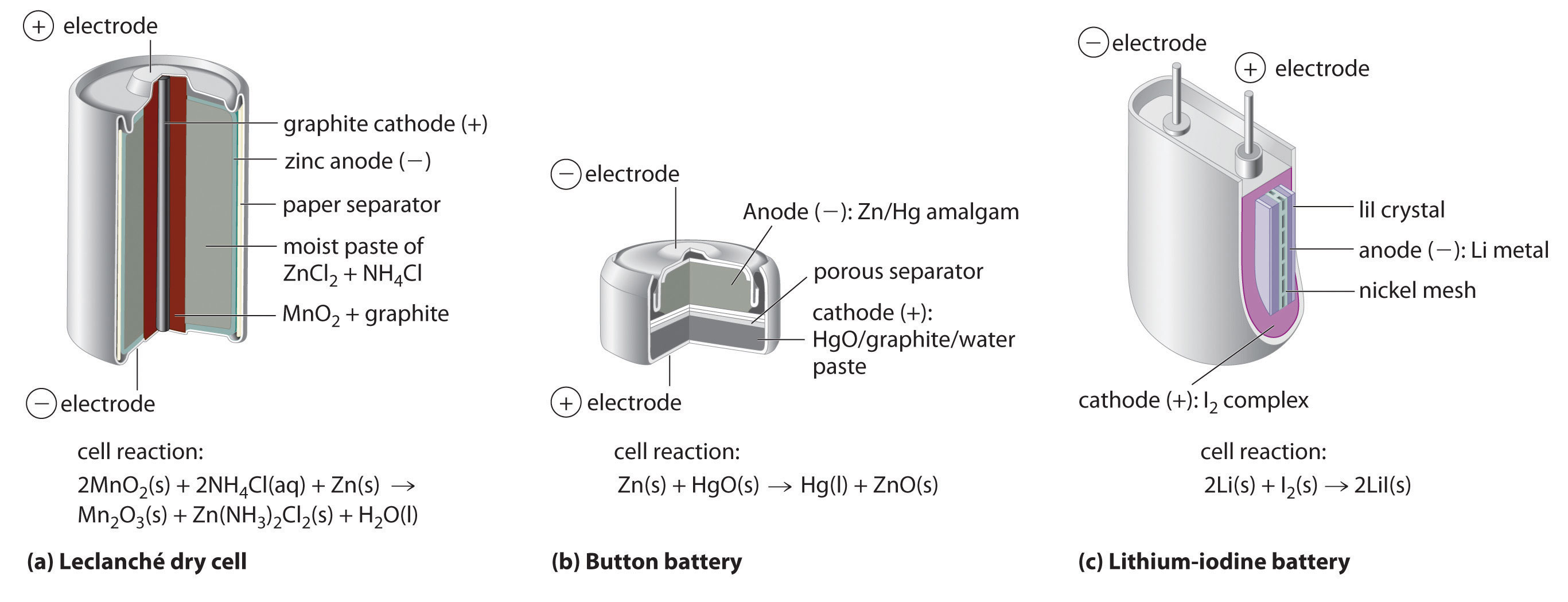
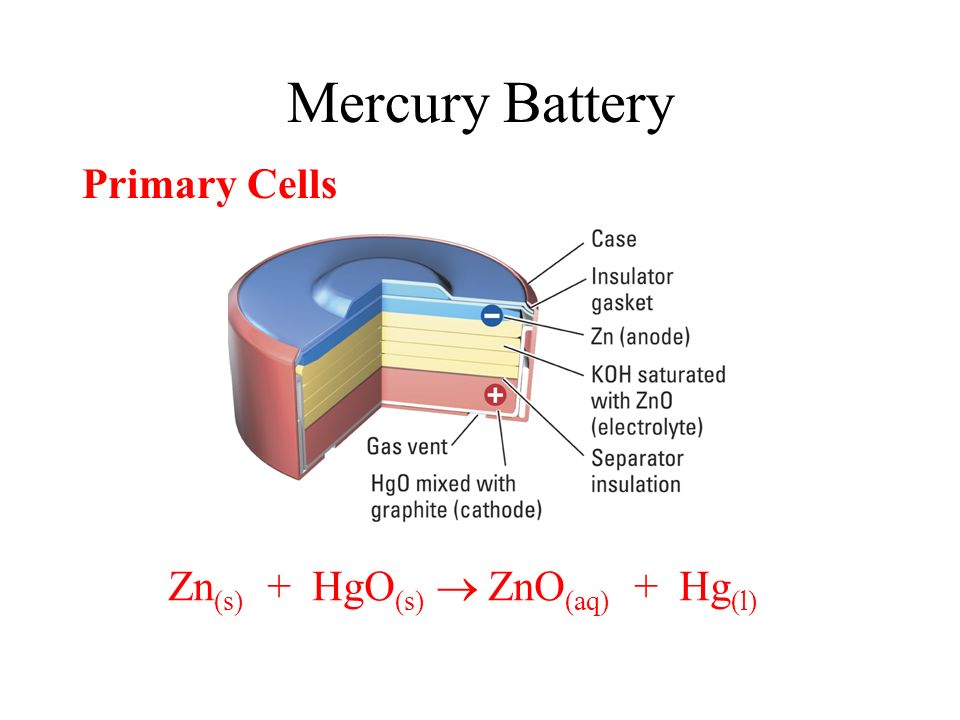
Redox Reactions and Electrochemistry Chapter 19. Applications of Oxidation-Reduction Reactions. - ppt download
Write the anode and cathode reactions occurring in a commonly used mercury cell. How is the overall reaction represented? - Sarthaks eConnect | Largest Online Education Community



.jpg)
/bar-chart-arranged-by-batteries-115805894-581909763df78cc2e8f79704-5c4a1ece46e0fb0001bba1e6.jpg)